Abstract

Background: Although blood transfusion is fundamental throughout the course of hematologic malignancy, acute myeloid leukemia (AML) patients requiring intensive chemotherapy are left at the edges of patient blood management programs because current guidelines do not have established recommendations for a transfusion threshold in patients treated for hematological disorders nor for those with severe thrombocytopenia who are at risk of bleeding. Especially in the past 2 years, patient blood management programs gained more popularity with the spread of COVID-19. Due to blood inventory shortages, majority of the institutions lowered the hemoglobin (Hb) threshold to 7g/dL and this hit patients with hematologic malignancies especially hard.
To close the gaps in inconsistencies of RBC transfusion care, we conducted a prospective trial focusing on newly diagnosed AML patients undergoing intensive chemotherapy. To the best of our knowledge, this is the first trial focusing on this unique subpopulation of patients. Also by doing so, we were able to shed some light on RBC transfusion optimization for patients with thrombocytopenia.
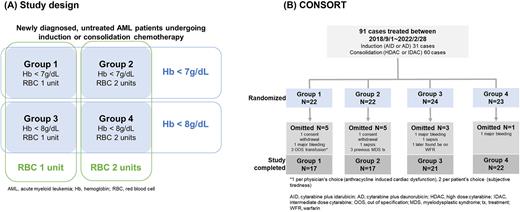
Methods: This was a randomized trial of newly diagnosed AML diagnosed and treated between September 2018 and February 2022. Figure A outlines the study design. Patients were randomized into 4 groups using 2 by 2 factorial design, according to trigger (Hb 7 vs 8g/dL) and quantity (single-unit vs double-unit). In case patients became hemodynamically unstable due to major bleeding, severe infection, ischemic heart disease, stroke or any other compromising conditions, liberal RBC transfusion was allowed regardless of the grouping to ensure patients’ safety. Platelet transfusion was done liberally to maintain target platelet count between 10 - 20 x 109/L. Non-acute promyelocytic leukemia AML aged between 19 to 70 years old, undergoing cytarabine ± anthracycline chemotherapy were considered eligible for enrollment.
The primary objective of the study was to compare the differences in the number of RBC units per case across the groups. The secondary outcomes included major bleeding and clinical relevant non-major bleeding (CRNM) according to ISTH, AML treatment outcomes according to International Working Group, platelet transfusion, RBC transfusion adherence rates (events of out of specification transfusion), transfusion related adverse events (AE), and infection complications
Results: Initially 91 patients were randomized into 4 groups, but the protocol adherence rate was 90.1% (Figure B). As whole, patients received a median of 4 units of RBC (range 0-24) and AE rate associated with RBC transfusion was 16.9% (13/77). All 13 cases of AE were mild allergic and non-hemolytic reactions. There were no cases of fatal AEs such as anaphylaxis, acute hemolysis or transfusion-associated lung injury.
Hemoglobin trigger did not affect the amount of RBC transfusion required during treatment. Patients receiving RBC transfusion at Hb < 7 g/dL used a median of 4 units of RBC (range 0-12), and those receiving transfusion at Hb < 8g/dL also used a median of 4 units of RBC (range 0-24) (P=0.305). The AE rates associated with RBC transfusion were similar between the 2 groups (P=0.286). As for platelet transfusion, there were no differences regards to transfusion frequency (P=0.256) or amount (P=0.258) between the 2 groups.
Quantity of RBC per transfusion did not affect the total amount of RBC transfusion required during treatment. Patients receiving 2 units of RBC per transfusion used similar amount of RBC as patients receiving 1 unit of RBC per transfusion. There were no differences in platelet transfusion frequency (P=0.256) or amount (P=0.258) between the 2 groups.
Overall, treatment outcomes did not differ across the 4 groups. There were 3 cases of major bleeding: 2 cases of gastrointestinal bleeding and 1 case of muscle hematoma. There was 1 case of CRNM: the patient had grade 1 epistaxis that did not require intervention. There were 4 deaths during the follow-up: 2 due to uncontrolled AML and 2 due to infection.
Conclusion: This study establishes feasibility for restrictive RBC transfusion in AML patients undergoing chemotherapy. For stable patients without significant medical history, RBC transfusion can be safely initiated when Hb < 7g/dL and 1 unit is adequate.
Disclosures
Koh:Sanofi Genzyme: Research Funding. Yoon:Takeda: Consultancy; Amgen: Consultancy; Chugai Pharmaceutical: Consultancy; Roche-Genetech: Research Funding; Yuhan Pharmaceutical: Research Funding; Janssen Pharmaceutical: Consultancy; Celgene: Consultancy; Astellas Pharma: Consultancy; Kyowa Kirin: Research Funding; Novartis: Consultancy; Tikaros: Consultancy.
Author notes
 This icon denotes a clinically relevant abstract
This icon denotes a clinically relevant abstract
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal